Clinical trials are the foundation of progress in cancer care. They determine which therapies are safe, effective, and ready to be integrated into standard treatment. Yet participation remains unequal. Only 2 - 5% of adult cancer patients successfully enroll in trials, with minority populations and patients from disadvantaged backgrounds consistently underrepresented [1, 2].
This underrepresentation threatens both the fairness and the accuracy of clinical research. Therapies validated in narrowly defined patient groups may not reflect the diverse populations seen in everyday oncology practice. Closing the participation gap is therefore essential to ensuring that all patients benefit from innovation.
Barriers to Inclusive Participation
Challenges to equitable enrolment occur across three dimensions:
-
Patient-related barriers: Lack of awareness of trial opportunities, logistical and financial obstacles such as travel or childcare, and mistrust in the healthcare system [1, 3].
-
Physician-related barriers: Time pressure, limited training in clinical research, and insufficient incentives. Busy clinicians often prioritize routine care over trial recruitment [1].
-
Industry-related barriers: Narrow eligibility criteria, complex regulatory requirements, and long consent documents that can overwhelm patients [1].
Inequalities Exposed
The pandemic highlighted longstanding disparities in trial access. While willingness to participate does not differ significantly across ethnic groups, barriers such as cultural mistrust, stigma, and inadequate outreach limit minority participation [2].
Similarly, socioeconomic status (SES) plays a decisive role. Patients with limited financial resources may find it difficult to afford transport, take time off work, or navigate complex study requirements [2]. Without addressing these obstacles, participation gaps persist regardless of patients’ interest in contributing to research.
These disparities are not abstract; but they are clearly reflected in FDA-approved cancer drug trials. The table below shows how overwhelmingly Caucasian the enrolment has been, leaving minority populations significantly underrepresented.
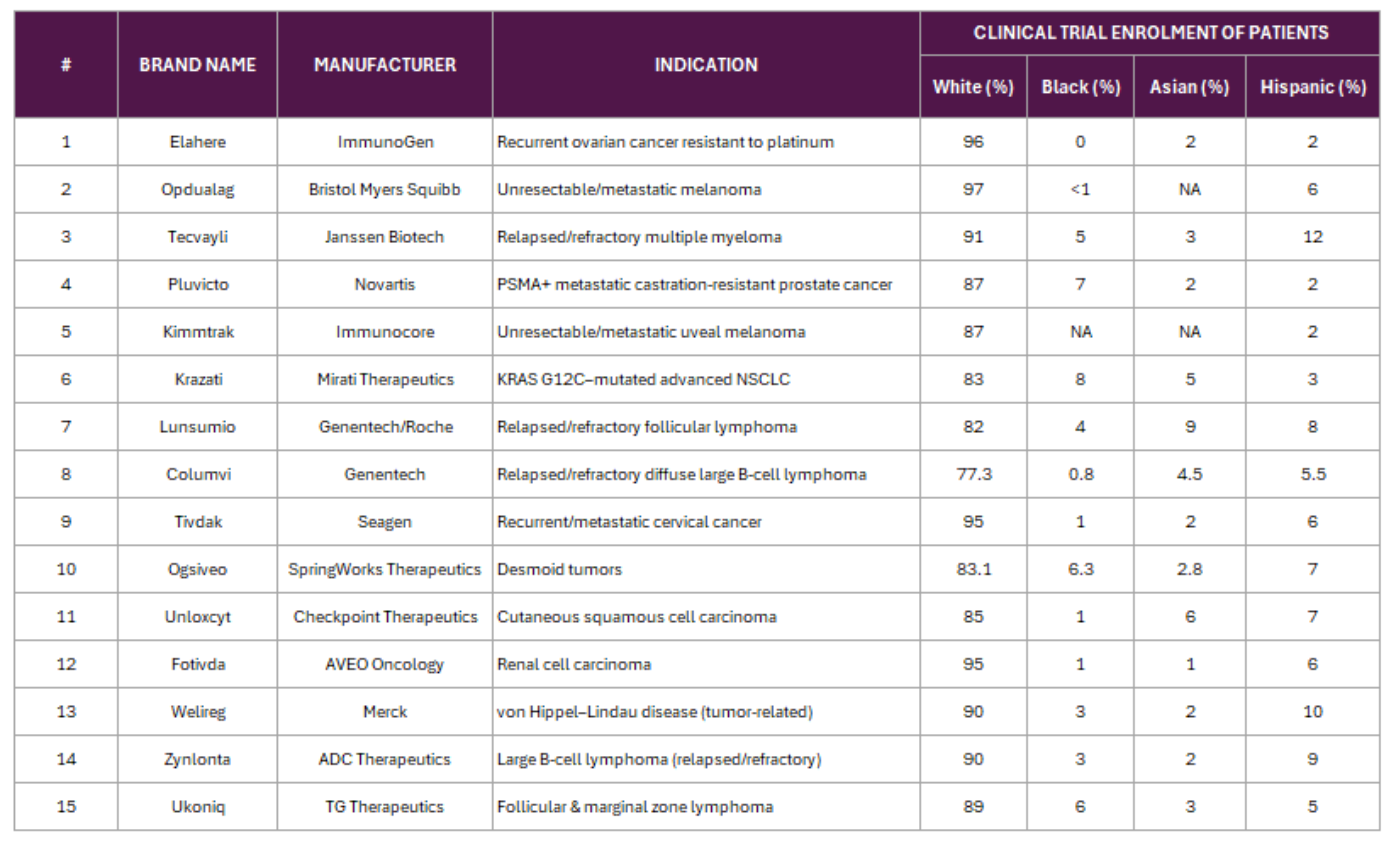
Table 1 Clinical Trials not showing real global diversity \@2025. PAICON All Rights Reserved [4]
Strategies to Close the Gap
-
Broaden Eligibility Criteria
Relaxing overly strict inclusion and exclusion criteria can expand access, reduce costs, and improve the real-world relevance of trial outcomes [1].
-
Enhance Cultural Competence
Training clinical and research staff in cultural awareness and patient-centered communication helps to build trust with diverse communities [2].
-
Embed Patient and Public Involvement (PPI)
Genuine collaboration with patient groups and community leaders ensures that recruitment strategies are relevant, accessible, and respectful [2].
-
Reduce Financial and Logistical Burdens
Providing travel reimbursement, flexible appointment scheduling, digital monitoring options, or childcare support makes participation feasible for more patients [2].
-
Bridge the Trust Gap
Transparent collaboration between investigators and industry partners can strengthen confidence and reduce delays caused by misaligned expectations [1].
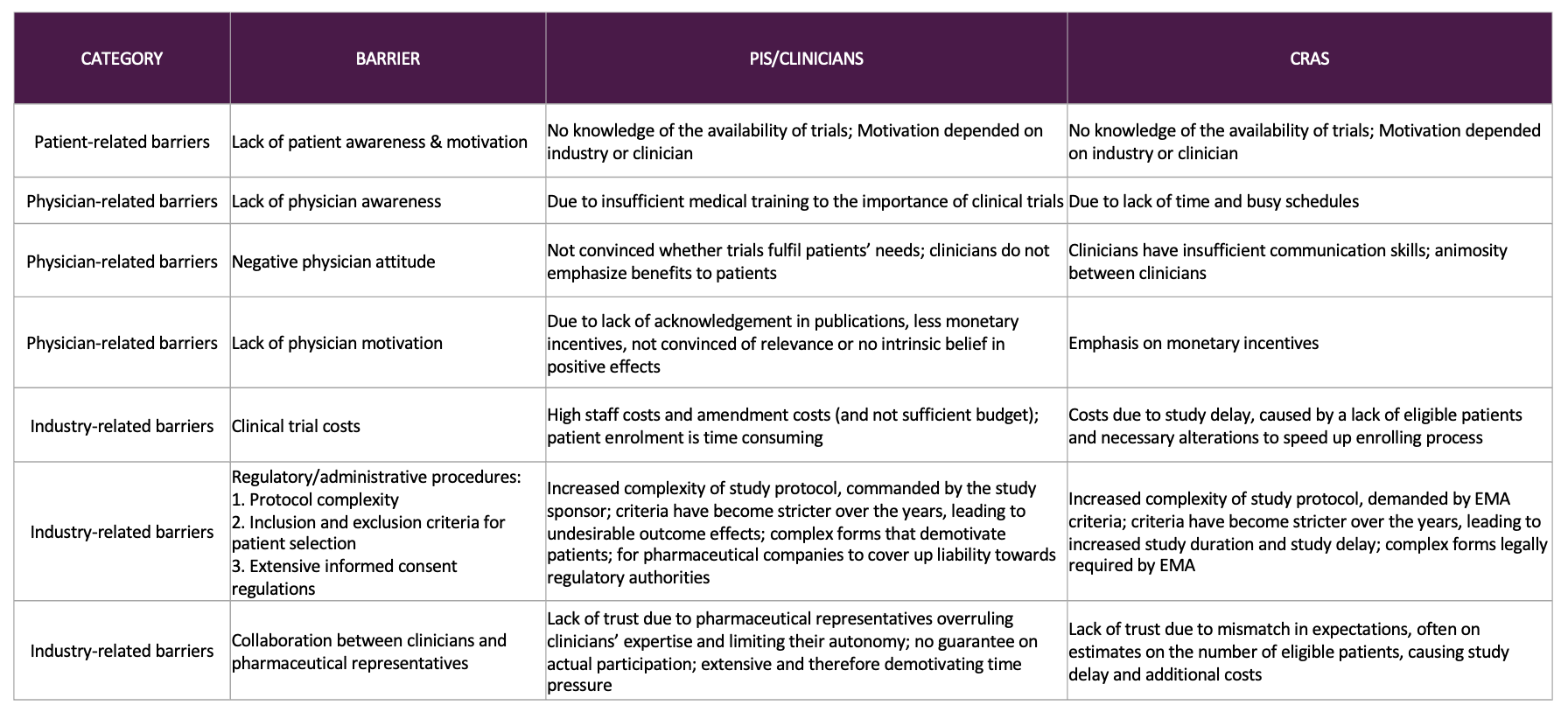
Figure 2: CRA, clinical research associate; EMA , European Medicines Agency; PI, principal investigator [1].
From Evidence to Action
The tools to improve equity are already available. Roadmaps, toolkits, and patient involvement frameworks provide clear guidance on designing inclusive trials [2]. What is needed now is consistent implementation: making equity a standard requirement rather than an optional consideration.
Embedding inclusivity from the earliest stages of trial design ensures that new therapies are tested, validated, and optimized for the diverse populations they are meant to serve.
Conclusion: Equity Is Not Optional
Equity in oncology trials is more than an ethical obligation; it is a scientific necessity. By breaking down barriers to participation, fostering cultural competence, and addressing structural inequalities, clinical research can deliver results that are more representative, more reliable, and ultimately, more impactful.
Closing the participation gap is essential to advancing cancer care and ensuring that tomorrow’s therapies benefit all patients.
References
-
Dane A, Ashraf S, Timmis J, et al. Barriers to patient enrolment in phase III cancer clinical trials: interviews with clinicians and pharmaceutical industry representatives. BMJ Open. 2022;12:e055165. doi:10.1136/bmjopen-2021-055165.
-
Pellizzari G. Closing the gaps in recruitment and retention in cancer trials: sufficient evidence but poor implementation. BMJ Oncology. 2023;2:e000195. doi:10.1136/bmjonc-2023-000195.
-
Byrne MM, Tannenbaum SL, Glück S, et al. Participation in cancer clinical trials: why are patients not participating? Med Decis Making. 2014;34(1):116–26. doi:10.1177/0272989X13497264.
-
Overview of diversity in clinical trial participation (based on FDA Drug Trials Snapshots Summary Reports, 2021–2024). Source: U.S. FDA, Center for Drug Evaluation and Research. Data retrieved on 4 November 2025.


